
Episodes

Monday Oct 09, 2023
Monday Oct 09, 2023
Dr. Nancy Lin at Johns Hopkins is working on a way to quickly diagnose Sarcoidosis - at last compared to the current ways it is being done. She is looking at our micro RNA to see if there is something different in sarcoidosis patients. Her research is being funded by a $150,000 grant from the Foundation for Sarcoidosis Research. Not only does it appear promising, but it may one day open doors to a cure. Listen to this fascinating conversation as Dr. Lin explores the root causes of sarcoidosis as far as we currently understand it.
Show Notes
- www.kinevant.com
- www.sarcoidosistrial.com
- Click here for information on how to sign up for the clinical trial: https://bit.ly/3DaVsR6
- ClinicalTrials.gov listing for RESOLVE-Lung: https://clinicaltrials.gov/ct2/show/NCT05314517
- ClinicalTrials.gov listing for RESOLVE-Heart: https://clinicaltrials.gov/ct2/show/NCT05351554
More on Dr. Nancy Lin https://www.stopsarcoidosis.org/fsr-awards-2022-2024-sarcoidosis-research-fellowship-to-dr-nancy-lin-of-national-jewish-health/
(Note: Dr Lin recently moved from National Jewish Health to Johns Hopkins)
Meet Olympic Cyclist Jennifer Valente: https://en.wikipedia.org/wiki/Jennifer_Valente

More on Kevin Moore's Sarc Battle: https://voice.vumc.org/a-mysterious-heart-ailment-almost-killed-kevin-moore-it-took-a-vanderbilt-team-to-pull-him-through/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser! If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com

Monday Sep 25, 2023
Monday Sep 25, 2023
Attorney Joel Rosen woke up one day and he wasn't feeling well. He thought maybe it was a cold. But he just couldn't get to feeling better. That sent him through a series of tests that eventually led to a diagnosis of sarcoidosis. As an attorney, he had already generously loaned his talents to several non-profits. And now we can add the Foundation for Sarcoidosis Research to the list. Joel is the newest board member for FSR, and in this episode of the Sarc Fighter podcast, he talks about how it took months to find a diagnosis and how he became more and more involved with the work FSR is doing to help patients cope, and researchers find treatments and maybe one day, a cure.

Show Notes
More on aTyr Pharma: https://atyrpharma.com/
Participate in the aTyr Clinical Trial: https://bit.ly/3EUOxNq
More on the Efzofitimod study: https://sarcoidosisnews.com/news/benefits-seen-efzofitimod-pulmonary-sarcoidosis-treatment-trial/
More on Joel Rosen: https://highswartz.com/high-swartz-news/sarcoidosis-board-member/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN:
John's Documentary from the U.S. Mexico border: https://www.wsls.com/video/news/2023/09/19/frontline-with-john-carlin--full-spec
John Carlin's Outdoors: https://www.wsls.com/topic/John_Carlin%27s_Outdoors/
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com

Monday Sep 11, 2023
Monday Sep 11, 2023
In Episode 96 of the FSR Sarc Fighter Podcast, fellow Sarc Fighter Royce Robertson returns to talk about his epic adventure while raising money for the cause. Meanwhile a small study in Japan suggests methotrexate may not be the answer for some Cardiac Sarcoidosis patients.
Show Notes
- www.kinevant.com
- www.sarcoidosistrial.com
- Click here for information on how to sign up for the clinical trial: https://bit.ly/3DaVsR6
- ClinicalTrials.gov listing for RESOLVE-Lung: https://clinicaltrials.gov/ct2/show/NCT05314517
- ClinicalTrials.gov listing for RESOLVE-Heart: https://clinicaltrials.gov/ct2/show/NCT05351554
The Methotrexate article: https://sarcoidosisnews.com/news/methotrexate-no-better-than-prednisolone-cardiac-sarcoidosis-poor-responders/
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
email Royce Robertson roycelrobertson@gmail.com
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser! If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com

Monday Aug 28, 2023
Monday Aug 28, 2023
Show Notes
More on aTyr Pharma: https://atyrpharma.com/
Participate in the aTyr Clinical Trial: https://bit.ly/3EUOxNq
More on the Efzofitimod study: https://sarcoidosisnews.com/news/benefits-seen-efzofitimod-pulmonary-sarcoidosis-treatment-trial/
Episode 77 interview with Dr. Christen Vagts https://beatsarc.podbean.com/e/episode-77-covid-vaccines-and-sarcoidosis-surprising-new-research-from-the-university-of-illinois-chicago/
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
John's Border Coverage: https://www.wsls.com/news/local/2023/07/24/videos-day-1-on-the-frontline-with-john-carlin-at-the-us-mexico-border/
John Carlin's Outdoors: https://www.wsls.com/topic/John_Carlin%27s_Outdoors/
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com
We all know how lousy sarcoidosis is. How it messes with our lives and makes us wish it wasn't there. It makes you wonder how you happened to be the one who was struck by lightning. (Listen on for more on that analogy!) Imagine a vacation where everything goes wrong -- or at least, not according to plans and dreams. Add in some significant health issues by family members and you have the makings of a good story! And a solid dose of perspective. This was my vacation this summer. Also, FSR has awarded Dr. Christen Vagts $150,000, to further her research is severe cases of pulmonary sarcoidosis. Dr. Vagts was a guest on the FSR Sarc Fighter podcast in December of 2022. There is a link to that episode below, if you would like to go back and listen.


John rides on the causeway from Burlington, Vermont. The best part of his vacation!

Monday Aug 14, 2023
Monday Aug 14, 2023
Tony Haskel first appeared on the podcast in March of 2023 - about five months prior to this recording. At the time he knew he had sarcoidosis, but he didn't really know what was in store. Now, months later he is much better acquainted with sarc, but still has an optimistic outlook.

Show Notes
- www.kinevant.com
- www.sarcoidosistrial.com
- Click here for information on how to sign up for the clinical trial: https://bit.ly/3DaVsR6
- ClinicalTrials.gov listing for RESOLVE-Lung: https://clinicaltrials.gov/ct2/show/NCT05314517
- ClinicalTrials.gov listing for RESOLVE-Heart: https://clinicaltrials.gov/ct2/show/NCT05351554
Support Tony's fundraiser! https://stopsarcoidosis.rallybound.org/TonyHaskel
Tony's email: tony.haskel@gmail.com
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser! If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com

Monday Jul 31, 2023
Monday Jul 31, 2023
In this episode of the FSR Sarc Fighter Podcast, I tested my health as I was embedded with the National Guard, patrolling the U.S. - Mexico border at Eagle Pass, Texas. Temperatures soared up to 103 degrees, as I worked to report on the battle between migrants wanting to cross into the United States, and authorities who want them to come through legally. It's a daily battle as the migrants cross the Rio Grande River, but come up against the concertina wire or C-wire barriers keeping them from crossing into Texas. In this Episode, I talk about what it was like to witness the stand off, as the heat and stress test my own strength and resolve as I deal with Sarcoidosis.

John on the border as a group of migrants enters the United States, near Eagle Pass, Tx.
Show notes:
More on aTyr Pharma: https://atyrpharma.com/
Participate in the aTyr Clinical Trial: https://bit.ly/3EUOxNq
More on the Efzofitimod study: https://sarcoidosisnews.com/news/benefits-seen-efzofitimod-pulmonary-sarcoidosis-treatment-trial/
Tony Haskins KISS fundraiser https://stopsarcoidosis.rallybound.org/TonyHaskel?tab=Dashboard
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
John's Border Coverage: https://www.wsls.com/news/local/2023/07/24/videos-day-1-on-the-frontline-with-john-carlin-at-the-us-mexico-border/
John Carlin's Outdoors: https://www.wsls.com/topic/John_Carlin%27s_Outdoors/
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com

Monday Jul 17, 2023
Episode 92 | Researcher Dr. Stephen Tilley on where we got sarcoidosis
Monday Jul 17, 2023
Monday Jul 17, 2023
Dr. Stephen Tilley is an Associate Professor of Medicine at the University of North Carolina. He specializes in lung disease with a concentrated effort on sarcoidosis. He is researching new methods and tools to combat the disease, by looking at the response our bodies create to different pathogens -- which could lead to a greater understanding of the cause and treatment of Sarcoidosis. In addition, Dr. Tilley believes that we may have inhaled something that ultimately causes our bodies to trigger the immune response that becomes sarcoidosis. In the interview, he will discuss his beliefs and why some people get sarcoidosis and others do not.

Show Notes
- www.kinevant.com
- www.sarcoidosistrial.com
- Click here for information on how to sign up for the clinical trial: https://bit.ly/3DaVsR6
- ClinicalTrials.gov listing for RESOLVE-Lung: https://clinicaltrials.gov/ct2/show/NCT05314517
- ClinicalTrials.gov listing for RESOLVE-Heart: https://clinicaltrials.gov/ct2/show/NCT05351554
More on Dr. Stephen Tilley: https://www.med.unc.edu/medicine/pulmonary/people/tilley/
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser! If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com

Monday Jul 03, 2023
Monday Jul 03, 2023
In Episode 91 of the FSR Sarc Fighter podcast, John shares two reports about developments in the treatment of sarcoidosis. He looks at recent studies published in Sarcoidosis News that show promise for aTyr Pharma's efzofitimod, currently in clinical trials, and also a report from Sweden that shows the difficulty in diagnosing neurosarcoidosis without an invasive and dangerous biopsy.
Show notes:
More on aTyr Pharma: https://atyrpharma.com/
Participate in the aTyr Clinical Trial: https://bit.ly/3EUOxNq
More on the Efzofitimod study: https://sarcoidosisnews.com/news/benefits-seen-efzofitimod-pulmonary-sarcoidosis-treatment-trial/
The study about neurosarcoidosis: https://sarcoidosisnews.com/news/few-patients-get-definite-neurosarcoidosis-diagnosis-study/
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
John Carlin's Outdoors: https://www.wsls.com/topic/John_Carlin%27s_Outdoors/
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com

Monday Jun 19, 2023
Monday Jun 19, 2023
 Matthew Campbell was trying to come back from a couple years of being out of shape, but his body seemed off somehow. His normal pep on the basketball court just wasn't there. What WAS there was sarcoidosis. Listen in as Matthew shares the story of how he gradually realized he was fighting way more than his fitness.
Matthew Campbell was trying to come back from a couple years of being out of shape, but his body seemed off somehow. His normal pep on the basketball court just wasn't there. What WAS there was sarcoidosis. Listen in as Matthew shares the story of how he gradually realized he was fighting way more than his fitness.
Show notes:
More on aTyr Pharma: https://atyrpharma.com/
Participate in the aTyr Clinical Trial: https://bit.ly/3EUOxNq
Back episodes from folks at the gala:
Erica Courtenay Mann: https://podcasts.apple.com/ca/podcast/episode-55-erica-courtenay-mann-has-sarcoidosis-on/id1499587273?i=1000551017276
Warren Robinson: https://beatsarc.podbean.com/e/episode-50-warren-robinson-s-father-died-suddenly-and-the-family-struggled-to-find-out-why/
Calvin Harris: https://www.youtube.com/watch?v=X1BJS2VMJSY
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
John Carlin's Outdoors: https://www.wsls.com/topic/John_Carlin%27s_Outdoors/
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com

Monday Jun 05, 2023
Monday Jun 05, 2023
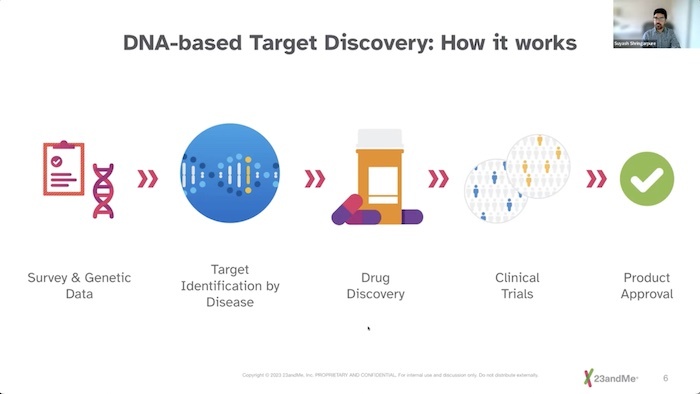
We don't know what causes Sarcoidosis, but we are getting closer and closer. A part of that research is coming from genetic experts at 23andMe. The genetics company has worked with thousands of people to study their DNA -- and to drill down to see if there is a connection between our genes and whether we get Sarcoidosis. Their findings were presented at an FSR Townhall forum hosted by Sarc Fighter Podcast Host John Carlin. The panelists include:
C. Ann Scott- Woman of Color Patient Advisory Committee, Foundation For Sarcoidosis Research, Dr. Courtney Montgomery- Director of the Sarcoidosis Research Clinic at the Oklahoma Medical Research Foundation, Mary McGowan – Chief Executive Officer, Foundation for Sarcoidosis Research, and Dr. Suyash Shringarpure – Senior Statistical Geneticist of 23andMe.
Show notes
Watch the Town Hall on YouTube. https://youtu.be/S2VLXmsTrBY
More about 23andMe https://www.23andme.com/
Royce's Cycle4Sarc page: https://stopsarcoidosis.rallybound.org/cycle4sarc?tab=Dashboard&fbclid=PAAaa9zWEjpGVyS1Q5Swa8mm5JT0t7JH13dfxVxdW1QlBMmbiRmc00Ol-uu-c
Royce Robertsons original interview: https://beatsarc.podbean.com/e/episode-79a-royce-robertson-is-fighting-sarcoidosis-from-the-seat-of-his-bike/
Help FSR further its mission by becoming an Alliance Volunteer: www.stopsarcoidosis.org/gsca-leaders/
Become a community outreach leader: https://www.stopsarcoidosis.org/gsca-leaders/
MORE FROM JOHN
Cycling with Sarcoidosis http://carlinthecyclist.com/category/cycling-with-sarcoidosis/
Do you like the official song for the Sarc Fighter podcast? It's also an FSR fundraiser!
If you would like to donate in honor of Mark Steier and the song, Zombie, Here is a link to his KISS account. (Kick In to Stop Sarcoidosis) 100-percent of the money goes to the Foundation. https://stopsarcoidosis.rallybound.org/MarkSteier
The Foundation for Sarcoidosis Research https://www.stopsarcoidosis.org/
Donate to my KISS (Kick In to Stop Sarcoidosis) fund for FSR https://stopsarcoidosis.rallybound.org/JohnCarlinVsSarcoidosis?fbclid=IwAR1g2ap1i1NCp6bQOYEFwOELdNEeclFmmLLcQQOQX_Awub1oe9bcEjK9P1E
My story on Television https://www.stopsarcoidosis.org/news-anchor-sarcoidosis/
email me carlinagency@gmail.com
email Royce Robertson roycelrobertson@gmail.com
Together they discussed the methods they use for the research as well as important issues of privacy, and the findings to date.
